Inflammation is a natural response to the formation of a wound, denoting that the body’s microbiological processes are actively trying to repair the ulcer site.
The uptick in microbiological activity appears as inflammation, redness and warmth around the wound.
However, excess, uncontrolled long-term inflammation is a sign that the body’s immune system is not functioning correctly.
When wound-healing stalls, it tends to be in the inflammation (rather than haemostasis, proliferation or maturation) phase.
Thus, one of the key challenges faced by clinicians when dealing with wound care is to be able to ‘jump start’ the stalled wounds that have stuck in the inflammatory stage of healing [1].
This is particularly true for people with diabetes, an underlying comorbidity that creates a high risk of not only developing a wound, but one that is at high risk for difficulties in healing.
A recent study shows that soluble beta-glucan (SBG) — a novel, bioactive, topical preparation — can restart healing in stalled wounds, including diabetic foot ulcers (DFUs) [2].
Healing issues posed by diabetes
Wound healing is dependent on the equilibrium between removal of damaged tissue and new tissue formation, a process that is regulated by the immune system’s production of growth factors and proteases [3].
Many of the factors that can delay wound healing — such as diabetes, malnutrition, impaired mobility, obesity and cardiovascular disorders — inhibit production, release and delivery of these factors.
Dysregulated inflammatory response is believed to be an important result of diabetes mellitus, which in turn can result in chronic, low-grade inflammation in wounds — that is, the wounds become ‘stuck’ in the inflammatory stage because the immune cells (most notably the macrophages) that regulate the balance of these factors are not performing as they should [2].
Furthermore, if the macrophages are not present or not performing their job in the wound bed, this can give rise to other factors that stall healing — such as microbial colonisation, excess wound exudate, and elevated levels of inflammatory cytokines, free radicals and proteases — which the body may be less able to combat due to the physiological changes brought about by diabetes [4].
Dysregulated cells from diabetic humans and animals can respond to stimulation or activation with beta-glucan, which can help attract other necessary cells to the wound bed and aid their effectiveness [5].
Clinical trial: soluble beta-glucan on diabetic foot ulcers
Beta-glucans have a long history of research that supports their role in promoting normal immune function which could, in turn, help move wounds out of the inflammatory stage and into the proliferation phase, so that angiogenesis, collagen deposition, granulation tissue formation, epithelialization, and wound contraction can occur [2].
In particular, SBG, the proprietary active ingredient in Woulgan Bioactive Beta-glucan Gel, helps activate macrophages which restart normal production of desirable growth factors and cell-signalling molecules, to promote the processes that move wounds towards closure and healing.
The randomised, double-blind, two-centre, placebo-controlled study by Zykova et al was undertaken to explore safety, tolerability and efficacy of SBG as a local treatment for DFUs.
Sixty patients with type 1 or 2 diabetes and lower-extremity ulcers (Wagner grades 1-2) received SBG or a standard-of-care product (methylcellulose) locally three times a week up to 12 weeks, in addition to conventional management.
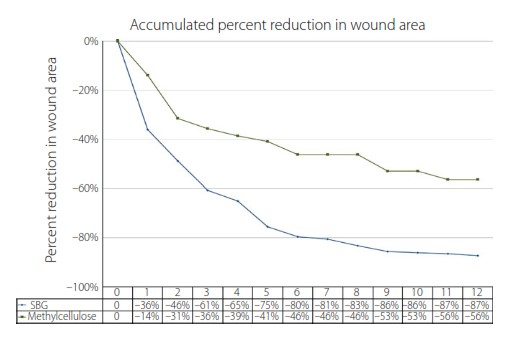
Median wound size was significantly smaller in the methylcellulose group at 2.87 cm2, compared to median 4.39 cm2 for the SBG group [2].
In the 54 patients who completed the study, wound surface areas reduced at a significantly faster rate for those in the SBG group (Figure 1).
Patients in the SBG group also experienced faster healing, and higher proportions of healing at 8 and 12 weeks than those treated with methylcellulose (Table 1).
| Table 1. Soluble beta-glucan versus methylcellulose for speed of healing[2] | ||
| Treatment with soluble beta-glucan treatment | Treatment with methylcellulose | |
| Median healing time | 36 days | 63 days |
| Proportion of ulcers healed by week 12 | 59% | 37% |
| Proportion of ulcers healed by week 8 | 44% | 17% |
In addition, SBG was safe and well-tolerated, with a clinically significantly lower incidence of serious adverse events as compared to methylcellulose [2].
Far-reaching implications
These results are important because of the increasing prevalence of type 2 diabetes, and the fact that 1 in 4 diabetes patients will develop a chronic wound as a result of diabetes at some point over their lives [6].
At any time, 4.4–10.5% of people with diabetes are being treated for DFUs [7].
The cost of treating chronic wounds is significant and growing. It’s estimated that, across Europe, 2–4% of healthcare expenditure is spent on wounds, and this figure is on the rise [8–10].
The average cost of treating a wound ranges from about €6,000–€10,000 per year [10].
In the UK, up to more than £5 billion (€6.4 billion) is spent on treating wounds annually, based on recent budget projections [11].
In particular, wounds in patients with diabetes are expensive to treat. The risk of amputation in diabetes is 23 times that of people without diabetes [12].
The cost of DFUs and amputations to the NHS in England alone in 2010–11 has been estimated at £639 million–£662 million, with approximately £1 in every £150 the NHS spent going towards managing DFUs and amputation [12].
Furthermore, patients with DFUs score significantly lower in quality-of-life measures — including social life and functioning, wellbeing with general health, physical symptoms, and daily living — than patients with healed ulcers [13].
And the presence of a DFU has the same negative affects across these measures, regardless of wound size [13].
This could be in part due to DFU-related pain, which can occur frequently and intensely even in the presence of peripheral neuropathy [14].
This experience has significant deleterious effects on patients physically and psychologically [14].
Decreased quality of life has further repercussions for the health system, as overall costs of care may increase, for example due to the need to provide ongoing psychological care [15].
Soluble beta-glucan: an important option for healing in stalled DFUs
Restarting healing in DFUs is, therefore, an acute need, not just for patient quality of life, but to improve cost-efficiencies of the healthcare system.
Based on the clinical trial, “SBG treatment promotes ulcer healing more rapidly, and the relative ulcer size reduction seems to be even more pronounced during the very first weeks of treatment,” write the authors.
“Thus, targeting and modulating macrophage function by an immunomodulator, such as SBG, seems to be a promising approach to promote healing of DFUs”. [2]
With significantly improved and faster healing rates, SBG-containing Woulgan has the potential to not only offer an effective, safe, well-tolerated, treatment for stalled wounds, including DFUs — but an economically sound option that meets both clinical needs of patients and financial goals of health systems.
References
- Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015;4(9):560-82
- Zykova SN, Balandina KA, Vorokobina NV, et al. Macrophage stimulating agent soluble yeast b-1,3/1,6-glucan as a topical treatment of diabetic foot and leg ulcers: A randomized, double blind, placebo-controlled phase II study. J Diabetes Investig. 2014;5(4):392-9.
- Li W, Chong S, Huaung E, Tuan T. Plasminogen activator/plasmin system. A major player in wound healing. Wound Rep Reg 2003; 11: 239-247.
- Cullen B, Ivins N. PROMOGRAN & PROMOGRAN PRISMA Made Easy. Wounds Int 2010;1(3):S1–S6
- Leibovich SJ, Danon D. Promotion of wound repair in mice by application of glucan. J Reticuloendothel Soc 1980;27(1):1–11.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005; 293: 217–28.
- Reiber GE, Vileikyte L, Boyko EJ, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999; 22: 157–62.
- Purwins S, Herberger K, Debus S, et al. Cost-of-illness of chronic leg ulcers in Germany. Int Wound J. 2010;7(2):97–102.
- Hjort A, Gottrup F. Cost of wound treatment to increase significantly in Denmark over the next decade. J Wound Care. 2010;19(5):173–4, 176, 178, 180, 182, 184.
- Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health-care providers in Europe. J Wound Care. 2009;18(4):154–161.1.
- Using Nanova Therapy System in Practice: a round table discussion. London: Wounds UK. 2015;11(4):Supplement
- Kerr M; Insight Health Economics. Foot care for people with diabetes: The economic case for change. Leeds, UK: NHS Diabetes, 2012. Accessed July 2016 at: https://www.diabetes.org.uk/Documents/nhs-diabetes/footcare/footcare-for-people-with-diabetes.pdf
- Jaska PJ, Mahoney JL. Quality of life in patients with diabetic foot ulcers: validation of the Cardiff Wound Impact Schedule in a Canadian Population. Int Wound J 2010;7(6):502-7
- Bradbury S, Price P. The impact of diabetic foot ulcer pain on patient quality of life. Wounds UK 2011;7(4):32-49
- Wounds International Expert Working Group. International consensus: Optimising wellbeing in people living with a wound. London, Wounds International 2012. Accessed July 2016 at: http://www.woundsinternational.com/media/issues/554/files/content_10309.pdf